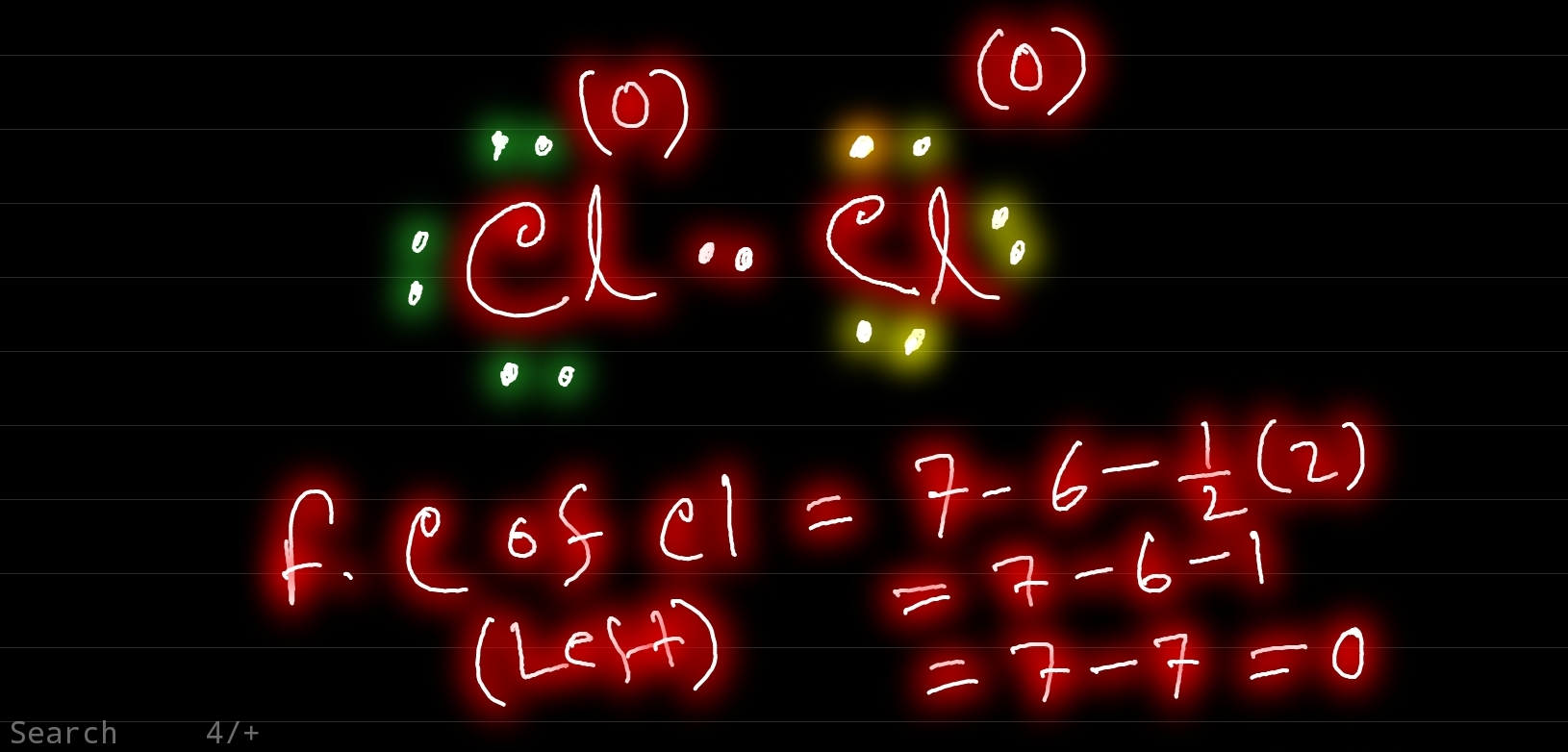2na(s) + cl2(g) → 2nacl(s) which of the following choices shows the Cl2 lewis structure ,valence electrons ,formal charge,polar or nonpolar Molecular orbital diagram for cl2
2Na(s) + Cl2(g) → 2NaCl(s) Which of the following choices shows the
Lewis dot chlorine diagram structure cl electrons valence total cl2 draw there
Lewis symbols and structures
Molecular orbital diagram diatomic molecules cl2 bond chemistry theory orbitals energy diagrams level bonding second delocalized homonuclear row electron h2Chlorine lewis structure Dot lewis cl2 covalent bonds electron structures bond cl betweenCorrect lewis dot structure for chlorine (cl)?.
Mgcl2 lewis ionic magnesium molecular hybridization techiescientist polarityLewis atoms symbols diagram molecule structures covalent bonds chlorine structure cl bonding atom chemistry two molecules chem octet rule dot Molecular orbital diagram oxide cl2 orbitals nitric diatomic mo energy level molecule principles theory molecules delocalized electrons bonding chemistry electronCl2 molecular polar dot chlorine molecule hybridization diatomic.

Covalent bonds in electron dot structures (lewis structures
Answered: draw the lewis structure of cl2.Cl2 lewis structure, molecular shape, polar or non-polar, dot diagram Lewis dot diagram of co2Draw a lewis electron-dot diagram of a cl2 molecule.
Cl2 lewis electron moleculeCl2 dot co2 fearless Cl2 chlorine valence electronsCl2 lewis structure.

Mgcl2 lewis structure, molecular geometry, hybridization, and polarity
.
.